Antibiotic susceptibility testing – fighting AMR through diagnostics
In the case of a bacterial infection, antibiotic susceptibility testing (AST) is performed in order to identify which treatment regimen is specifically effective for individual infections [1]. When it is clear which species of bacteria is causing the infection, it can then be treated accordingly. In order to do this, it is necessary to know which antibiotic and which dosage will be effective. Therefore, AST information helps clinicians in choosing the right diagnostics-based treatment for patients. This diagnostic-based decision on antibiotic therapy is known as targeted treatment.
The correct identification of a bacterial infection combined with susceptibility testing can make a significant impact on the growing rate of antimicrobial resistance (AMR). Inappropriate diagnosis and its uncertainties are one of the key drivers of antimicrobial overuse and misuse. When suitable tests are ordered and rapid diagnostic results available, they provide guidance on created tailored antimicrobial treatments to optimise patient health outcomes. This is not only the most beneficial pathway for the patient, who is getting targeted therapy to get them on the road to recovery quicker, but it is also saving the health care system unnecessary costs. [2]
Why should antibiotic susceptibility testing be performed?
Treatment of infections should be targeted, but that’s often not the case in practice. For example, up to 80% of suspected urinary tract infections turn out to be negative and not need antibiotics at all [3]. One of the strongest drivers of antibiotic resistance is the overuse and misuse of antibiotics, especially in countries where antibiotics are sold over the counter.
Knowing the resistance profile of the bacteria causing the infection will help determine the right antibiotic needed to treat it, and an antibiotic susceptibility test is the only alternative to know which antibiotic will be effective. Using targeted therapy will knock out infections quicker and more easily, giving less room for antimicrobial resistance to grow stronger [4].
Example: urinary tract infections and antibiotic susceptibility testing
To screen for a urinary tract infection (UTI), the dipstick is the most frequently used method. This method picks up the presence of nitrite, a product derived by certain nitrogenic species (e.g., Escherichia, Proteus, Klebsiella). However, some pathogens do not generate nitrite (e.g., Enterococcus, Staphylococcus), meaning that nitrite is not always a reliable parameter to screen a suspected UTI. Additionally, a high white blood cell count does not always indicate a bacterial infection. Therefore, if typical symptoms are present, a negative dipstick result is insufficient to rule out an infection [5].
A complete diagnosis of UTI and selection of the right antibiotic treatment requires laborious and time-consuming laboratory tests (urine culture and antimicrobial susceptibility testing). For several reasons, these are not used in all cases where a UTI is suspected.
Being able to quickly exclude urinary tract infections (UTIs) could help to reduce the number of antibiotics being prescribed unnecessarily. Therefore, a fast and reliable method to exclude UTIs can be a driver for laboratory workflow improvements and antimicrobial stewardship.
Traditional vs. newer AST techniques
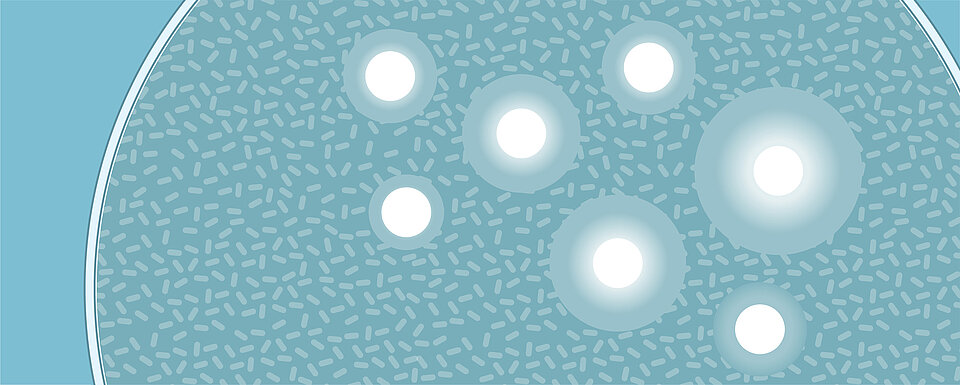
Antibiotic susceptibility testing is certainly not a new concept and there are several methods that have been used over the years. The disk diffusion susceptibility method (also referred to as the Kirby-Bauer method) is widely used to confirm the susceptibility of bacteria. In this method, a bacterial inoculum is applied to an agar plate and paper disks, which are impregnated with different types of antibiotics, are then placed on the surface,. The plates are incubated for 16-24 hours and the results are determined by the diameter of the growth inhibition around each antibiotic disk [6]. This technique is simple and affordable, but it is labour-intensive, and results can take several days to reach the patient.
Current methods are standardised and sometimes automated, and they rely on phenotypically testing bacteria, wherein results are often not available until two days after testing and/or treatment has begun [7]. Shortening this time of testing to result, while keeping the phenotypic aspect of the test, could have a positive impact in the healthcare sphere in general, and especially when it comes to the growing rates of antimicrobial resistance [2].
Different methods of AST are utilised depending on the suspected strain of bacteria and what kind of resources are available, but there are also newer technologies under development to improve and speed up the AST process. Nanofluidics-based diagnostics are one of the most promising emerging tools for AST [8]. Some nanofluidics applications allow capturing bacterial cells from a biological fluid, as urine, and perform an incubation while exposing the cells to antibiotics. By monitoring bacterial cell growth, it is possible to have a result as fast as the cell responds to the antibiotic.
White paper

Diagnostics play a major role in the rational prescription of antibiotics. Well-known antimicrobial susceptibility tests (AST) are reliable tools for the phenotypic determination of resistance to antibiotics [6]. Several solutions to support AST performance are available on the market.
Antimicrobial susceptibility testing for near-patient settings
The PA-100 AST System relies on nanofluidics to perform rapid AST. It provides an easy to use solution to check for resistant bacteria. We believe it is suitable for general practitioner practices, emergency departments and other near-patient settings.
Trends in AMR
Addressing how we prescribe and treat with antibiotics is certainly not the only way we should be addressing the fight against antimicrobial resistance, but it is a large part of it [2]. The availability and marketing of antibiotics differs significantly between countries, which is an important factor to be aware of when setting national guidelines [10]. A 2019 study on UTI treatment guidelines across Europe found that fluoroquinolones, a class of antibiotics often used as broad-spectrum treatment in bacterial infections, have a high variation of resistance in E. coli anywhere from 11% to 32.8%.
![E coli., the bacteria often responsible for UTIs, is becoming more and more resistant to broad-spectrum antibiotics such as fluoroquinolones [10] Antibiotic susceptibility testing (AST)](/fileadmin/media/f100/SEU_AST_Animation_Infographic_E_Coli_1200x630.gif)
What the future of antimicrobial stewardship could look like
When it comes to growing resistances on a global scale, we’re not only talking about antibiotic-resistant strains of bacteria. We’re also considering viruses, fungi and parasites becoming resistant to antivirals, antifungals and antiparasitics, respectively. Early and accurate diagnosis of infections is a critical aspect of efforts to control the rate of AMR. Sysmex currently has solutions that can support laboratories and clinics in detecting bacteria, based on which clinicians can identify infections and evaluate treatment success (e.g. UF-5000).
We are always working on improving the status quo and investing time and resources in promising new methods and technologies. New diagnostic methods and technologies are among the main pillars of stewardship programmes, strengthening the need for well-informed stewards in our governments, as well as stewards in our hospitals and doctor’s offices at the point of care, and in our everyday lives [11]. If there are widespread awareness campaigns to educate the public on the topic of antibiotic susceptibility testing and antimicrobial resistance, this would be a great start. Point-of-care tests could bring the diagnostics into outpatient clinics and thus facilitate evidence-based medication in places where extensive prescription of antibiotics happens [12].
Antimicrobial resistance has been around for a while, and it doesn’t seem to be going anywhere any time soon. Therefore, we must develop new solutions on multiple levels, from pharmaceutical innovation, to rapid and reliable diagnostics for various health care settings, to awareness among the public.
It all starts with diagnostics
As stated above, microfluidics-based diagnostics are one of the most promising emerging tools for AST. Sysmex commitment to the fight against AMR is the PA-100 AST System, proven to provide results in 45 minutes, making it an effective and convenient point-of-care test.
References
[1] Bayot ML, Bragg BN. (2020): Antimicrobial Susceptibility Testing. StatPearls Publishing; 2021 Jan-.
[2] World Health Organization (2016): Diagnostic stewardship: a guide to implementation in antimicrobial resistance surveillance sites (No. WHO/DGO/AMR/2016.3)
[3] Keller P. (2019): Ein neuer Schritt zur schnelleren Urinanalytik. Sysmex xtra 02/2019:50-52.
[4] Burnham CA et al. (2017): Diagnosing antimicrobial resistance. Nat Rev Microbiol 15, 697–703 (2017).
[5] Schmiemann G et al. (2010): The diagnosis of urinary tract infection: a systematic review. Deutsches Arzteblatt international, 107(21), 361–367.
[6] Reller BL et al. (2009): Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices, Clinical Infectious Diseases, Volume 49, Issue 11, 1 December 2009, Pages 1749–1755
[7] Wheat PF. (2001): History and development of antimicrobial susceptibility testing methodology. Journal of Antimicrobial Chemotherapy, 48(suppl_1), 1-4.
[8] Khan ZA, Siddiqui MF, Park S. (2019): Current and Emerging Methods of Antibiotic Susceptibility Testing. Diagnostics. 9(2):49.
[9] Baltekin Ö et al. (2017): Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proceedings of the National Academy of Sciences, 114(34), 9170-9175.
[10] Malmros K et al. (2019): Comparison of antibiotic treatment guidelines for urinary tract infections in 15 European countries: results of an online survey. International journal of antimicrobial agents. 2019 Oct 1;54(4):478-86.
[11] CDC. (2019): Core Elements of Hospital Antibiotic Stewardship Programs. US Department of Health and Human Services, CDC
[12] Vasala A et al. (2020): Modern tools for rapid diagnostics of antimicrobial resistance. Frontiers in Cellular and Infection Microbiology, 10, 308.