Tracking cancer with liquid biopsy – more accuracy, less invasion
This article is taken from European Biopharmaceutical Review July 2022, pages 20-23. © Samedan Ltd
Did you know that the performance of tissue biopsies was already being done over 1,000 years ago in the 10th century? This first report on the use of needles for therapeutic purposes was found in Arab medicine, where Albucasis described puncturing the thyroid gland of a patient using instruments which resembled modern aspiration needles (1). Tissue biopsies are still performed today, however, biomarkers found in blood are increasingly gaining momentum in clinical care as they can provide similar information – and more easily, therefore more frequently – than a tissue biopsy (2).
The first liquid biopsy technology was developed at the Johns Hopkins School of Medicine by a research team that used a digital PCR (dPCR) technique known as BEAMing (3). Since then, many developments have been made in the detection of cancer biomarkers in liquid biopsies. One of the most important was the advancement of next generation sequencing (NGS) technologies which allow us to screen for a broader range of cancer mutations as well as increase the number of biomarkers and samples that are measurable per liquid biopsy assay.
How does liquid biopsy work?
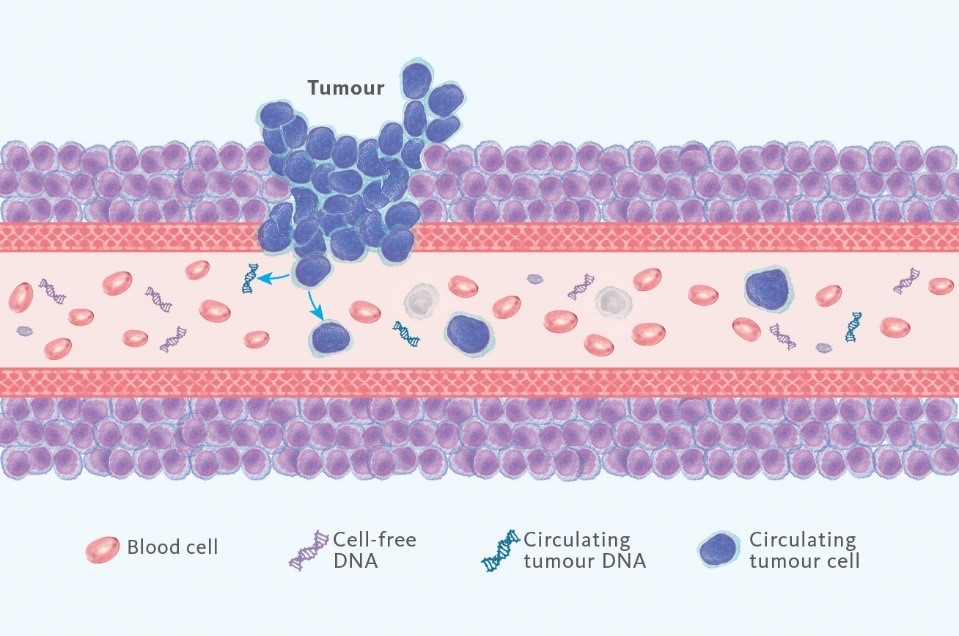
A liquid biopsy is a simple and minimally invasive alternative to surgical tissue biopsies, primarily utilising a blood sample. Simply put, in the case of cancer, tumour cells leave behind traces of mutated genes which can be found in the blood. Analysing these alterations has been increasingly used for diagnostic, prognostic and treatment purposes, with impressive advancements happening in the last couple of years (4).
Can liquid biopsy replace other cancer-detecting methods entirely? At least at the moment, tissue biopsies and liquid biopsies will coexist because they each provide benefits that the other lacks. Tissue biopsy has time and research on its side – it has nearly a millennium of recorded data and research to back up its benefits. However, there are cases where sampling is challenging or not possible. Additionally, many cancers have multiple genetic mutations and they may not have the same mutations in all parts of the cancer. When it comes to tissue biopsy, it may not reveal all mutations due to the limited sample volume and more complex sampling procedure. Liquid biopsy offers an improved chance of detecting these genetic changes, especially because they better reflect tumour heterogeneity. It is able to capture the genomic tumour make-up that is currently present in the blood, thus revealing tumour evolution, which is not visible in an initial tissue biopsy. Furthermore, in the case of liquid biopsy and the simplicity of the sampling procedure, a larger number of patients can be tested and with greater frequency.
More precision, less invasion
With just a sample of blood, it is possible to derive a lot of information about a tumour. Traces of DNA from the tumour are shed in the blood (called circulating tumour DNA or ctDNA) and the possible gene mutations are reflected in the liquid biopsy results. This can be used to help plan treatment, monitor how well treatment is working or detect a potential relapse after treatment.
There are several benefits to utilising liquid biopsy where it is applicable. It has the potential to detect an increase in the ctDNA levels in blood months, or even up to a year, before you can visibly see a recurrence of cancer using imaging (5). That means a quicker treatment response can be initiated to get a patient back to remission status.
Another positive aspect, both for clinicians and patients, is that liquid biopsy can be performed routinely due to its minimally invasive nature. For patients, this means fewer invasive procedures like tissue biopsies. For clinicians and researchers, this means they have a meaningful comparison of ctDNA data over time and can therefore identify groups of patients who are at risk of remission earlier than with standard methods, such as imaging.
Needle in a haystack
Although some researchers have been saying that liquid biopsy has the potential to be a gamechanger in cancer management, it doesn’t come without caveats – at least at this point in time. Diagnostic sensitivity can be a difficult hurdle when it comes to liquid biopsy because some patients may have very small amounts of ctDNA, making it very hard to detect. Furthermore, it is challenging to detect ctDNA among the high levels of DNA originating from tissue of non-tumour origin (4). In that way, it’s like looking for a needle in a haystack.
Additionally, and potentially the most important aspect for clinicians, pathologists and patients alike is the workflow and time to results. Many liquid biopsies are not performed locally, but rather at centralised labs. Due to this, results can take quite some time to come back and finally reach the patient. Those tests that are performed locally are often performed using in-house developed tests which might lead to a high degree of lab-to-lab variation due to lack of standardisation around liquid biopsy procedures and bioinformatic analysis.
Sysmex and liquid biopsy
There are emerging liquid biopsy techniques and tests that address the issues described above, and Sysmex is excited to be on the forefront with their newest IVD kits. Sysmex Inostics, a subsidiary of Sysmex Corporation, is a molecular diagnostic company that is a pioneer in blood-based cell-free tumour DNA mutation detection in oncology utilising ultra-sensitive technologies such as BEAMing (digital PCR) and SafeSEQ (NGS). In the EMEA region, they have now launched the Plasma-SeqSensei™ (PSS) Breast Cancer IVD Kit and the Plasma-SeqSensei™ Solid Cancer IVD Kit – the former is intended specifically for use in breast cancer and the latter in other solid cancers. These kits enable the ultra-sensitive and quantitative detection of mutations in ctDNA from blood plasma. In addition, fast turnaround time of two days and featured IVD-certified software for convenient data analysis and reporting are aimed at reducing the time from sampling to results.
Beyond mutant allele fractions – absolute mutant molecule quantification
The quantity and quality of tumour-derived DNA in a given sample can vary dramatically, therefore high-analytical sensitivity over a broad dynamic range is necessary for the detection of ctDNA (4). PSS kits offer ultra-high sensitivity – the breast cancer kit has a sensitivity down to 0.06% mutant allele fractions (MAF) and absolute limit detection of six mutant molecules, and the solid cancer kit has a sensitivity down to 0.07% MAF and absolute limit detection of seven mutant molecules. This is important for clinical research purposes such as response monitoring, recurrence monitoring and minimal residual disease detection.
The amount of cell-free DNA (cfDNA) in any given sample can vary by patient and external factors such as exercise (6). With other liquid biopsy technologies, the results are typically calculated by quantifying the amount of ctDNA against the entirety of cfDNA to get the MAF. But because external factors can affect the amount of cfDNA, it can be difficult to compare results over different time points. In the PSS IVD kits, an independent quantifier called Quantispike is added to the sample. This enables absolute quantification of the ctDNA molecules which can then be reported as number of mutant molecules detected in the tested volume of blood, like for any other blood biomarker, facilitating meaningful comparison of liquid biopsy results over time.

Bringing liquid biopsy to the NGS level
Some researchers challenge NGS-based liquid biopsy as being less sensitive than dPCR-based solutions. For Sysmex Inostics, it was important to base their new products on a technology that will allow NGS-based liquid biopsy to reach dPCR sensitivity. Here is where the SafeSeq™ technology came in handy. This technology, like the abovementioned BEAMing, originates from Johns Hopkins University and uses unique molecular identifiers (UID) that are introduced during the first step of the protocol. This results in the formation of UID ‘families’ consisting of various copies of each UID assigned, allowing for the discrimination of real mutations from errors that may be introduced during the complicated amplification and sequencing process. Thanks to UIDs, PSS kits offer a 100-fold lower error rate in comparison to the standard NGS-based liquid biopsy protocols.
The PSS Breast Cancer IVD Kit covers six gene targets (AKT1, ERBB2, ESR1, KRAS, PIK3CA, TP53) while the PSS Solid Cancer IVD Kit covers five gene targets (BRAF, EGFR, KRAS, NRAS, PIK3CA). These genes significantly contribute to the development of their respective cancer types and are important biomarkers utilised for prognosis as well as monitoring of recurrence and therapy response (7). Additionally, the PSS Solid Cancer Kit supports clinicians in analysing the RAS mutation status to determine the potential benefit of anti-epidermal growth factor receptor (EGFR) therapy for colorectal cancer patients.
Calculating and understanding results
What would an innovative test kit be without an accompanying software to match? The PSS IVD Software offers the possibility to effortlessly plan and analyse sequencing runs and report data easily. It is the first IVD-certified, NGS analysis software designed to run on an office PC or laptop – no workstation or server structure is needed. The interface is divided into three functional modules: run planning, data analysis and reporting. Automatically generated reports include sample metrics and mutation status, both listing MAFs as well as the absolute number of mutant molecules detected in the sample volume, alongside sequencing quality metrics. The comprehensive report is designed for clinicians, featuring a clear layout highlighting sample mutation status and vital QC parameters.
Liquid biopsy within Sysmex’s portfolio
Prior to this newest product launch, Sysmex brought BEAMing technology to the market in 2008 with the OncoBEAM™ kits. This product line enabled 60 clinical studies to be performed with more than 40,000 samples analysed, often in collaboration with pharmaceutical companies. This was the perfect springboard to develop the Plasma-SeqSensei™ IVD Kits.
Sysmex is proud of an ever-growing portfolio of life science solutions used throughout the pathway of cancer care in prevention, detection, diagnosis and monitoring. The mission is to support pathologists and oncologists by delivering essential genetic information, as quickly as possible. With profound scientific knowledge and an understanding of clinical needs, we work closely with partners to deliver innovative diagnostic solutions and therapeutic support that address patients and their therapy individually. Not all cancers and patients are alike, therefore specific diagnostic methods are vital in facing these challenges together.
Liquid biopsy is a technique that is not yet implemented widely, but it is gaining ground as an innovative solution in cancer detection, recurrence surveillance and therapy response monitoring. Every medical advancement needs early adapters who are willing to take the leap and see for themselves how these innovations can benefit not only the people handling treatment, but most importantly the patients. Will you be one of them?
References
- Diamantis, A et al. (2009): Fine-needle aspiration (FNA) biopsy: historical aspects. Folia Histochemica et Cytobiologica, 47(2), 191-197.
- Nature Research Custom Media & illumina. As liquid biopsy technology improves, cancer research stands to benefit. www.nature.com/articles/d42473-021-00324-y
- Dressman, D et al. (2003): Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proceedings of the National Academy of Sciences, 100(15), 8817-8822.
- Crowley, E et al. (2013): Liquid biopsy: monitoring cancer-genetics in the blood. Nature reviews Clinical oncology, 10(8), 472-484.
- Misale, S et al. (2012): Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature, 486(7404), 532–36.
- Velders, M et al. (2014): Exercise is a potent stimulus for enhancing circulating DNase activity. Clinical biochemistry, 47(6), 471-474. www.sciencedirect.com/science/article/pii/S0009912013006024
- Brandão, M & Sotiriou, C. (2019): Multigene Sequencing in Breast Cancer: ESMO Biomarker Factsheet. Education, 37(10K), 1.