Scientific Calendar January 2019
How early can highly sensitive liquid biopsy detect resistance to targeted therapy in non-small cell lung cancer patients compared to conventional imaging?
4 weeks
8 weeks
12 weeks
> 13 weeks
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific Background Information
Not only have treatment options evolved dramatically, but the diagnostic workup in non-small cell lung cancer (NSCLC) has also undergone important changes over recent years.
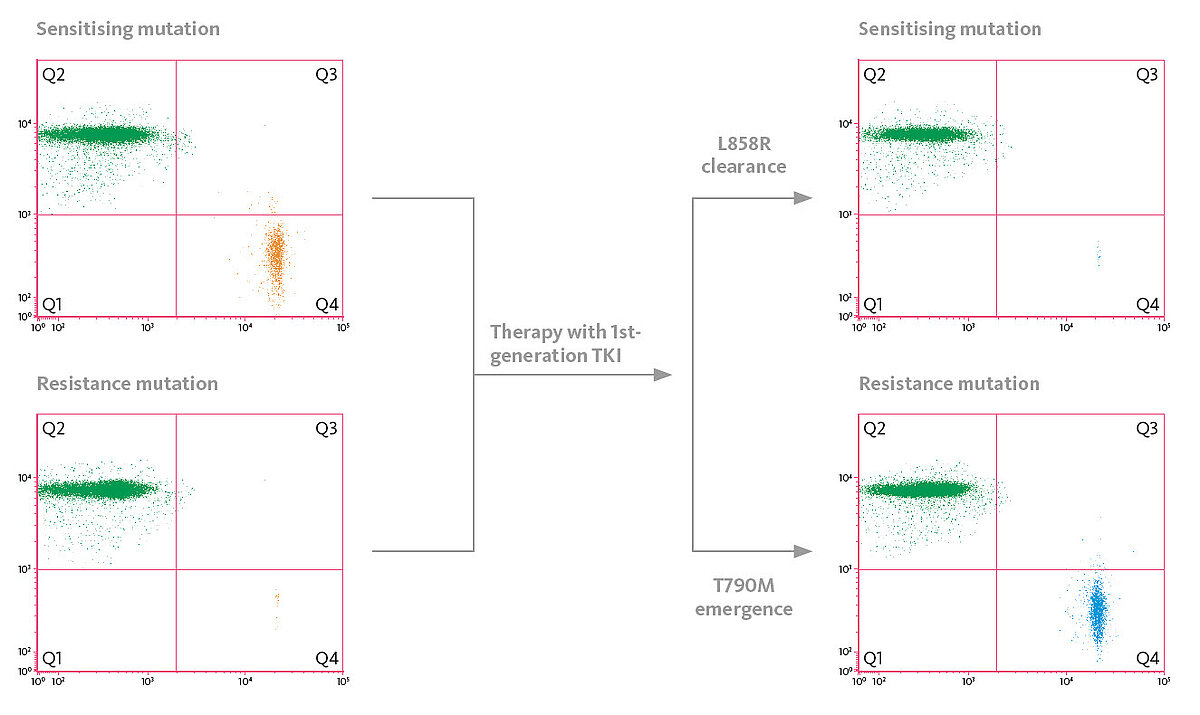
Today it is well-understood that upon treatment with 1st or 2nd line tyrosine kinase inhibitors (TKI), T790M, the most common EGFR resistant mutation, is commonly detected and signals the need to change treatment to the 3rd line TKI osimertinib. To be able to adapt therapy, one needs to analyse treatment-relevant mutations during TKI treatment, but usually there is no tissue available to monitor the mutational status sequentially. Therefore, liquid biopsy is the key to a more sophisticated understanding of a patient’s condition. Circulating tumour DNA (ctDNA) released from the tumour to the bloodstream can add essential information for precision medicine in NSCLC patients and allow for early adaptation of treatment strategies.
Dynamic changes of certain mutations during TKI treatment can be indicative of treatment response or resistance early on in NSCLC patients. Recently, data from the prospective multicentric LungBEAM study have been presented where NSCLC patients have been followed up during TKI therapy (1). Due to the high sensitivity of the OncoBEAM EGFR assay used for mutational analysis, the investigators have been able to detect emerging EGFR T790M mutations 13.6 weeks prior to radiological progression assessed by imaging. Furthermore, clearance of EGFR mutations of plasma 8 weeks after initiation of TKI therapy may be an indicator of a more favourable progression-free survival.
In other tumour entities, OncoBEAM liquid biopsy also demonstrated the potential to monitor cancer patients during treatment. In colorectal cancer, several studies have shown the possibility of early detection of resistance or response to anti-EGFR therapy (2). In a subset of melanoma patients, ctDNA signalled disease progression based on BRAF mutations an average of 21 weeks earlier than radiographic imaging (3). Furthermore, when serial blood samples from pancreatic cancer patients were evaluated up until tumour progression, KRAS-mutant ctDNA proved superior at indicating disease progression over CEA or CA 19-9 measurements (4).
Taken together these promising data clearly demonstrate the need and power of precision diagnostics for precision medicine in cancer care where being flexible in treatment strategies really matters.
Scattergrams
References
(1) Garrido, P et al. LungBEAM: A prospective multicenter trial to monitor EGFR mutations using BEAMing technology in Stage IV NSCLC patients. Ann. Oncol. 2018, Volume 29, Issue suppl_8, 1, mdy269.119
(2) Garcia-Foncillas J et al. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann. Oncol. 2017 0(0):1 – 7.
(3) Rowe, SP et al. From validity to clinical utility: the influence of circulating tumor DNA on melanoma patient management in a real-world setting., Mol Oncol. 2018 Oct;12(10):1661-1672.
(4) Kruger, S. et al. Repeated [mut]KRAS ctDNA measurements represent a novel and promising tool for early response prediction and therapy monitoring in advanced pancreatic cancer. Ann. Oncol. 2018, mdy417
OncoBEAM EGFR kit v2 (RUO) and OncoBEAM BRAF kit (RUO) are for research use only. Any in vitro diagnostics purpose has not been established by the manufacturer.