Scientific calendar April 2022
Irritable bowel syndrome and inflammatory bowel disease: sensitive testing for the detection of intestinal inflammation
What is special about calprotectin measurements with CALiaGold for the differential diagnosis of inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and during the monitoring of IBD patients?
Highly sensitive test for the detection of intestinal inflammation and therefore aiding in the differential diagnosis of IBD and IBS
Can be used for monitoring and optimising therapy in IBD patients
Can predict IBD relapses early on
Improved calprotectin stability in CALiaGold buffer enables home sampling
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
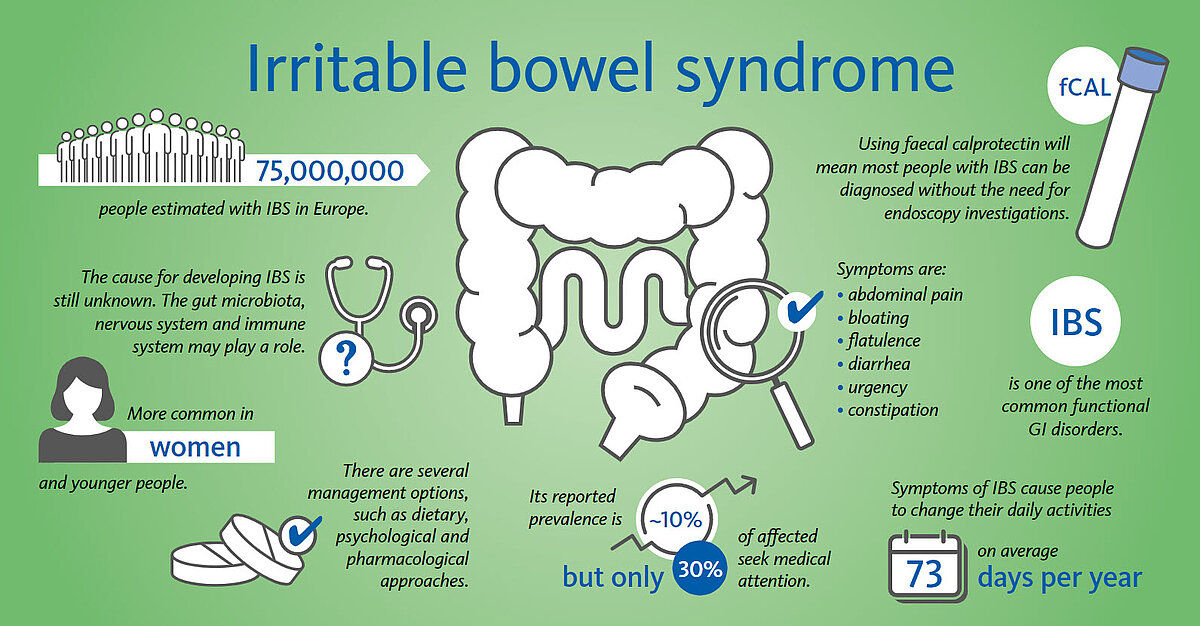
Irritable bowel syndrome (IBS) is one of the most common disorders of gut–brain interactions and is estimated to affect around 1 in 10 people globally (~75 million in Europe) [1]. IBS is a disorder in which a person experiences chronic, recurrent bowel problems and abdominal pain. Bowel problems may include constipation, diarrhoea, pain or a combination of these. A person with IBS will experience intestinal discomfort daily, but the frequency and severity of IBS symptoms are not predictable and can vary. If IBS symptoms are not managed, it can be disruptive to a person’s life. IBS can affect a person physically and emotionally. While the cause of IBS is not known, it is thought that the symptoms of IBS are brought on by a disruption of the interaction between brain, nervous system and gut. IBS cannot be cured, however, once medical assistance is sought and the condition managed, IBS may give minimal or no disruption to a person’s life.
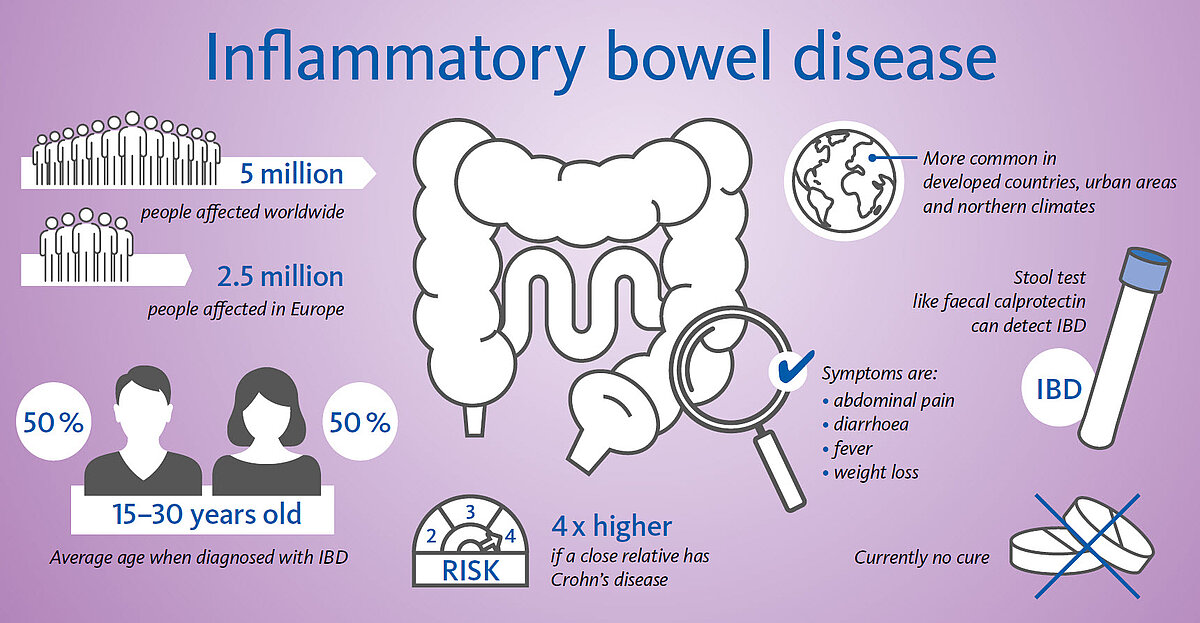
IBD, which stands for inflammatory bowel disease, includes Crohn’s disease (CD) and ulcerative colitis (UC), which are two serious, chronic digestive diseases that affect 5 million people worldwide (2.5–3 million in Europe [3]) [2]. Latest published incidence data has shown increasing numbers of IBD (6.8 million people worldwide [4]). Men and women are equally likely to be affected, and while the disease may occur at any age, IBD occurs more often among adolescents and young adults between the age of 15 and 35. IBD is a chronic inflammatory disease of the gut, which presents with periods of inflammatory activity (flares) and quiescent phases (remission) as can be seen with many chronic diseases. The majority of patients suffering from IBD in Europe experience a relapsing course of the disease whereas 20–25% of the patients experience chronic, continuous symptoms. Up to 30–40% of CD patients in Europe present with a complicated disease phenotype at the time of diagnosis, and a similar proportion of the patients may develop complications over the next 10–15 years during the follow-up phase [3]. There is no cure, no known cause, and little public understanding of the pain and chronic suffering with which IBD patients courageously cope every day of their lives. In most cases the illness can be kept under control with medication. Although there is no cure for IBD, its symptoms and impact on a patient’s life can be minimised by appropriate medical management.
Calprotectin is a protein mainly found in the cytoplasm of neutrophilic granulocytes. In cases of gastrointestinal (GI) inflammation, neutrophils migrate through the intestinal wall and calprotectin is released due to degranulation. It accumulates in the faeces and is excreted from the body. The concentration of calprotectin directly correlates with the number of neutrophils in the intestinal lumen, making it an ideal biomarker for GI inflammation. The determination of faecal calprotectin (fCal) is a non-invasive option to assess localised inflammation. Being highly sensitive for the detection of GI inflammation, it helps in the differential diagnosis of inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS). The biomarker fCAL outperforms general inflammation markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) since it is more sensitive and more specific. The aim of treating IBD is to secure and maintain remission, with the goal of achieving mucosal healing. fCAL levels seem to correlate with the severity of IBD, can predict IBD relapses and can be used for monitoring and optimising the therapy in IBD patients. Calprotectin values < 50 μg/g are not indicative of inflammation in the gastrointestinal tract. Patients with low calprotectin levels most likely do not need invasive procedures to determine the cause of inflammation. Calprotectin values between 50 and 200 μg/g can represent mild organic disease such as inflammation caused by mild diverticulitis and IBD in a remission phase. Calprotectin values > 200 μg/g are indicative of active organic disease with inflammation in the gastrointestinal tract, therefore appropriate additional investigative and curative procedures by specialists are recommended. Besides this, fCAL can be effective in managing colonoscopy resources. Calprotectin testing represents a non-invasive and accurate approach and can be a prognostic tool for clinical relapses.
CALiaGold is the complete solution for the automated quantitative determination of calprotectin in human faeces. The particle-enhanced turbidimetric immunoassay (PETIA) is based on the agglutination reaction between latex particles coated with antibody and the antigen in solution. It can be run, along with the FOB Gold test, on your dedicated faecal analyser (SENTiFIT 270 or SENTiFOB analyser).
The new version of CALiaGold has an improved sensitivity (LoB 3.2 µg/g, LoD 6.1 µg/g, LoQ 18.6 µg/g on the SENTiFIT 270), a prolonged tube stability (tube storage for several months at room temperature; samples in the tube buffer for 3 days at up to 37°C or for 14 days at 2–8°C) and a better performance with highly concentrated samples (prozone-free up to 6,000 µg/g).
Patients can easily collect the stool sample at home with the dedicated CALiaGold tube and bring or send it back to the GP/laboratory. This represents a more hygienic procedure for laboratory personnel and reduces their workload, as the tubes can be directly placed onto the dedicated analyser for processing. In addition, home sampling may prevent calprotectin from degradation during transportation as it is protected in the tube buffer solution.
So far, several CALiaGold-related method comparison studies have been published [5-10].


Irritable bowel syndrome and inflammatory bowel disease: essential facts
References
[1] Lovell RM et al. (2012): Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10:712–721.
[2] Burisch J et al. (2015): The epidemiology of inflammatory bowel disease. Scandinavian Journal of Gastroenterology 50(8):942–951.
[3] Burisch J et al. (2013): The burden of inflammatory bowel disease in Europe. Journal of Crohn's and Colitis 7:322–337.
[4] Naghavi M et al. (2020): The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5:17–30.
[5] Paparella C et al. (2018): Evaluation of a new assay for the quantitative determination of calprotectin in human feces (CALiaGold). UEG week poster presentation (P1721).
[6] Finazzi S et al. (2018): Comparison between two automated assays for the quantitative determination of fecal calprotectin. ELAS 2018 poster presentation.
[7] Silva B et al. (2019): Method comparison of CALiaGold (a new assay for quantitative determination of fecal calprotectin) and BÜHLMANN fCAL turbo. EuroMedLab 2019 Barcelona poster presentation.
[8] Rasmussen H et al. (2019): Comparison of two automated assays and extraction techniques for analyzing fecal calprotectin. IBD Nordic conference Malmö poster presentation (p11).
[9] Huijgen J et al. (2020): Analytical performance of the CALiaGold Quantitative latex immunoassay for Calprotectin measurement on the SENTiFIT 270 analyzer. NVKC Papendal (P18).
[10] Mérida F et al. (2020): Comparative Study of Three Different Fecal Calprotectin Immunoassays: TriCAL Study. Annals of Clinical & Laboratory Science 50(6):55–60.
Resources