Scientific Calendar March 2023
Test strip screening for albuminuria partly replacing quantitative technology
Why is frequent monitoring of risk group patients for chronic kidney disease important?
CKD is reversible at any stage
CKD affects 10% of the global population
CKD is not affected by increasing incidences of obesity
CKD progression can be slowed or stopped by early diagnosis and treatment
CKD is mainly caused by diabetes and hypertension
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
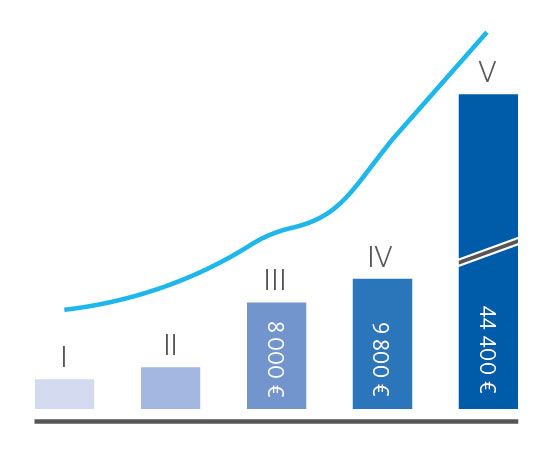
Chronic kidney disease (CKD) is defined as ‘persistent abnormalities of the kidney structure and/or function, present for more than three months’ [1] with hypertension, diabetes, and obesity being the main risk factors for the onset and progression of CKD. Since the prevalence of these civilisation diseases has been steadily increasing since the 1990s [2], an increase of the global prevalence of CKD to more than 10 % is not surprising. Besides its individual effects on patients’ quality of life, CKD is putting a significant burden on healthcare expenditures and the society [3]. In Germany, for instance, individual annual costs for treating a patient at stage five reaches an amount of € 45,000 [4] (Fig. 1). Considering the prevalence of CKD and underlying civilisation diseases, frequent screening of risk group patients for the onset of CKD is often recommended by international guidelines, but in reality hardly performed.
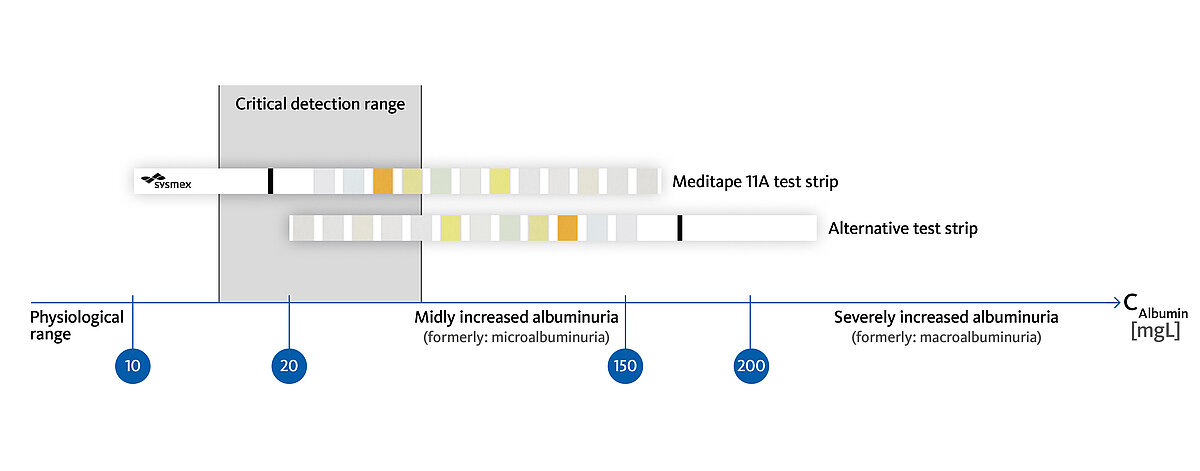
Apart from the estimated glomerular filtration rate (eGFR), urinary albumin is a key indicator for the development of CKD. It is essential to distinguish between mildly and severely increased albuminuria. While the early detection of albuminuria at a mildly increased level allows reverting the onset of CKD, severely increased albuminuria is irreversible and the course towards end-stage renal failure is set [5] (Fig. 2).
The test strip-based detection of albuminuria on the UC-3500 using Meditape UC-11A test strips has already been demonstrated to be comparable to quantitative approaches, such as immunonephelometry. Especially the low limit of detection of 5.5 mg/L allows a clear separation between physiological albumin levels and mildly increased albuminuria (Fig. 2) [6]. Considering the comparatively low costs for test strip-based techniques, economic considerations might no longer prevent a broad screening approach for CKD among risk group patients.
Depicted in the calendar image: diabetic nephropathy
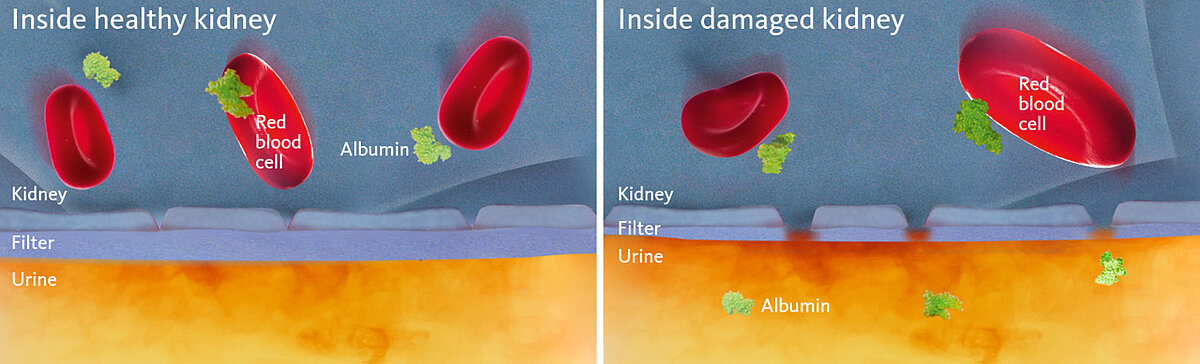
Under normal conditions, albumin remains in the blood and does not pass the glomerular filtration barrier consisting of endothelial cells of the glomerular blood vessels, the glomerular basement membrane (GBM), and podocytes. In uncontrolled diabetes, elevated blood glucose levels lead to increased levels of glucose in the primary urine, which in turn cause podocytes to produce reactive oxygen species (ROS). ROS then cause apoptosis of the podocytes and a breach of the glomerular filtration barrier, finally leading to albumin passing into the urine (albuminuria) (Fig. 3) [7].
Fighting occult chronic kidney diseases – and more
Can a test strip-based approach replace quantitative techniques for the early detection of albumin to allow screening of risk group patients for the onset of CKD?
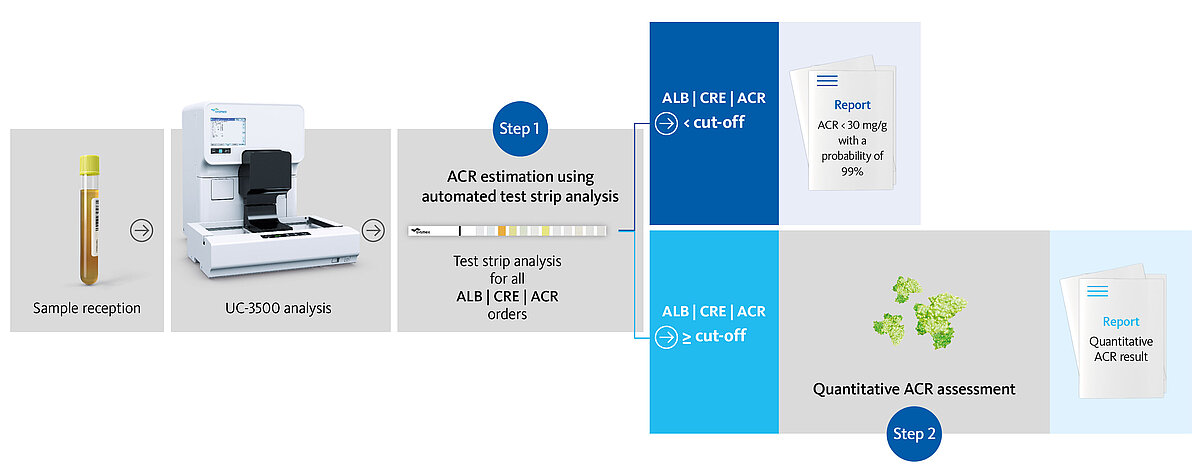
At the University Hospital San Juan (Alicante, Spain), María Salinas and her team investigated the potential of the automated test strip analysis on the UC-3500 to replace quantitative tests. Here, a new workflow was concluded (Fig. 4), with test strip analysis as the default screening for albuminuria. Upon exceeding defined cut-off values (≥ 10 mg/L albumin and ≥ 4.42 mmol/L creatinine), samples are suspicious of an increased albumin:creatinine ratio (ACR) and are finally subjected to quantitative assessment [8].
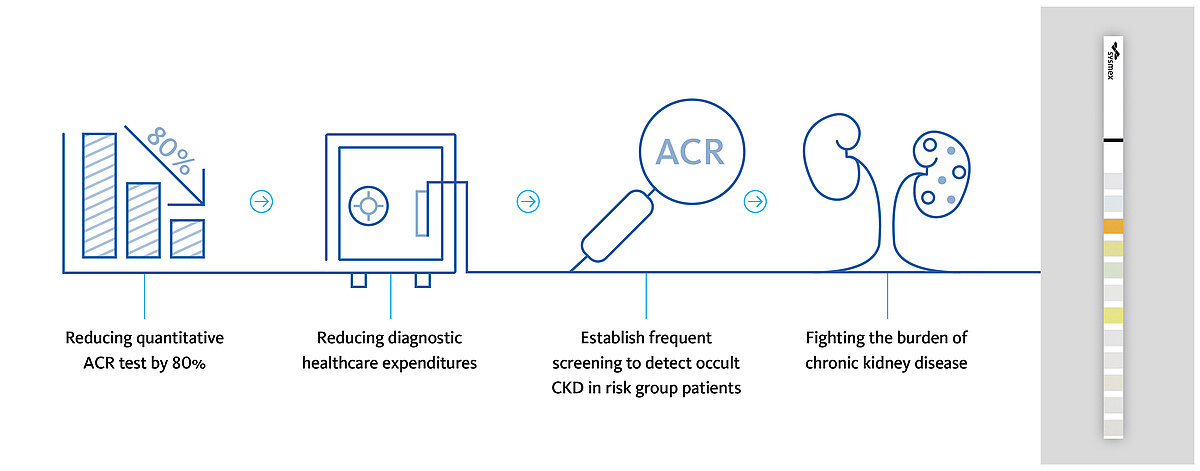
Meanwhile, the laboratory was able to reduce the need for quantitative assessments of albuminuria by up to 80 %, resulting in remarkable cost savings. These savings are directly converted into additional capacities for screening risk group individuals for the onset of CKD. This allows a wider-ranging early detection of CKD, fostering the fight against the burden of CKD (Fig. 5).
However, the laboratory at the University Hospital San Juan does not stop at the level of CKD but uses its cost savings also for screening for other occult diseases, such as diabetes. The early detection of these diseases allows early treatment to revert or at least slow down disease progression, with a positive impact on public health and social costs.
Here, screening is performed using defined algorithms that consider patient history, public health data and clinical practice guidelines, lifting laboratory diagnostics to the next level – not only by highlighting the importance of laboratory diagnostics, but also by strengthening the role of the clinical laboratory as a hub for decision-making [9].
References
[1] KDIGO (2012): Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter. Suppl. 2013; 3:1–150.
[2] Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H and Al Kaabi J (2020): Epidemiology of Type 2 Diabetes – Global Burden of Disease and Forecasted Trends. Journal of Epidemiology and Global Health 10(1):107–111.
[3] GBD Chronic Kidney Disease Collaboration (2020): Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733.
[4] Gandjour A, Armsen W, Wehmeyer W, Multmeier J and Tschulena U (2020): Costs of patients with chronic kidney disease in Germany. PLoS ONE 15(4):e0231375.
[5] Decavele AS, Fiers T, Penders J and Delanghe JR (2012): A sensitive test strip-based albuminuria screening assay. Clin Chem Lab Med 50(4):673–678.
[6] Delanghe JR, Himpe J, De Cock N, Delanghe S, De Herde K, Stove V and Speeckaert MM (2017): Sensitive albuminuria analysis using dye-binding based test strip. Clin Chim Acta 471:107–112.
[7] Reidy K, Kang HM, Hostetter T and Susztak K (2014): Molecular mechanisms of diabetic kidney disease. Journal of Clinical Investigation 124(6):2333–2340.
[8] Salinas M, López-Garrigós M, Flores E, Lugo J, Leiva-Salínas C and PRIMary Care-LABoratory (PRIMLAB) working group (2018): Urinary albumin strip assay as a screening test to replace quantitative technology in certain conditions. Clin Chem Lab Med 57(2):204–209.
[9] Salinas M, López-Garrigós M, Flores E, Martín E and Leiva-Salínas C (2021): The clinical laboratory: a decision maker hub. Clin Chem Lab Med 59(10):1634–1641.