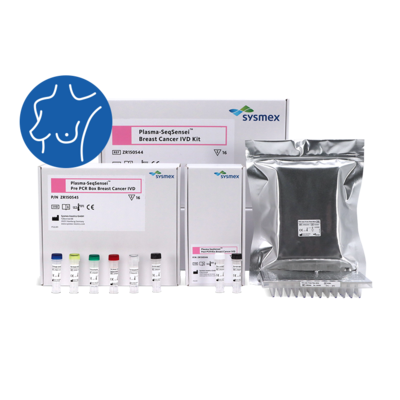
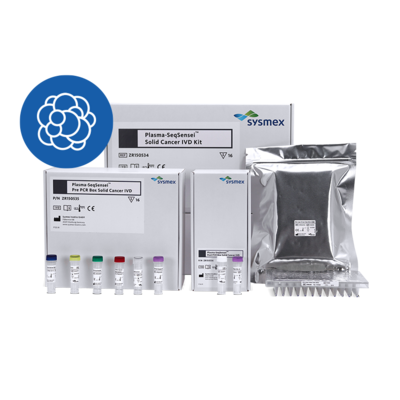
Sysmex Inostics, a global leader in blood-based circulating tumour DNA (ctDNA) analysis, together with Sysmex Europe SE, announce the introduction of two IVD-certified Plasma-SeqSensei™ (PSS) kits to the EMEA region. The Plasma-SeqSensei™ Breast Cancer IVD Kit is intended specifically for use in breast cancer and the Plasma-SeqSensei™ Solid Cancer IVD Kit is intended for use in other solid cancers. This release follows the successful launch of similar products as research use only (RUO) kits.
The PSS is a diagnostic test which allows for ultra-sensitive and quantitative detection of mutations in ctDNA from blood plasma. PSS IVD kits enable clinicians to monitor ctDNA levels in patients undergoing (neo-)adjuvant therapy and also support them in recurrence surveillance and in detecting minimal residual disease. In addition, the Solid Cancer IVD kit is intended to aid the clinician in analysing the RAS mutation status to determine the potential benefit of anti-epidermal growth factor receptor (EGFR) therapy for colorectal cancer patients.
“By naming the kits Plasma-SeqSensei™, we wanted to underline the sample type (plasma), the technology (Next Generation Sequencing, NGS) but also to show that this is a transferable technology, which can be mastered (Sensei = master) by anyone with laboratory training – including analysis and reporting part – to bring NGS-based liquid biopsy to the clinics and to patients,” says President and CEO of Sysmex Inostics, Dr. Bhuwnesh Agrawal.
High sensitivity delivered by PSS kits is achieved by a unique technology developed at Johns Hopkins University, which allows for the reduction of sequencing errors by >100 fold and confident detection of very small amounts of mutant DNA.
By providing high-quality, CE-IVD certified liquid biopsy tools, Sysmex Inostics together with Sysmex Europe continue their mission to support pathologists and oncologists by delivering essential genetic information, on time, facilitating effective treatment adaption and monitoring of cancer patients. For more information on how we are innovating in the field of liquid biopsy, visit www.sysmex-europe.com/liquidbiopsy
About Sysmex Inostics GmbH
Sysmex Inostics (President and CEO: Bhuwnesh Agrawal), a subsidiary of Sysmex Corporation, is a molecular diagnostic company that is a pioneer in blood-based cell-free tumour DNA (ctDNA) mutation detection in oncology utilising ultra-sensitive technologies such as BEAMing (digital PCR) and SafeSEQ (NGS). With more than 10 years’ experience in liquid biopsy, Sysmex Inostics is a trusted partner to leading pharmaceutical companies, advancing their efforts to bring the most effective personalised cancer therapies to global markets, from discovery through companion diagnostics. Discover more here: https://sysmex-inostics.com/
About Sysmex Europe SE
Sysmex supports healthcare professionals around the world in lighting the way with diagnostics by providing a broad range of medical diagnostics products and solutions. In the fields of haematology, urinalysis, haemostasis, life science, flow cytometry, essential healthcare and now immunochemistry, we combine highly dependable, multi-functional and easy-to-operate instruments, a variety of reagents and software, plus reliable service and support.
Sysmex Europe SE, located near Hamburg, Germany, is a subsidiary of the Sysmex Corporation from Kobe, Japan. From our Hamburg offices, we serve our affiliates, distributors and customers throughout Europe, the Middle East, and Africa (EMEA). For more information, visit www.sysmex-europe.com.