On July 1, 2020, Sysmex Corporation (HQ: Kobe, Japan; Chairman and CEO: Hisashi Ietsugu) began providing a lab assay service for research on cytokines,1which have been suggested as a useful indicator in monitoring the risk of increasing severity and treatment effects for the novel coronavirus (SARS-CoV-2), which causes the novel coronavirus disease COVID-19. Sysmex plans to continue conducting R&D with a view to expanding target parameters for lab assay and providing reagents for research.
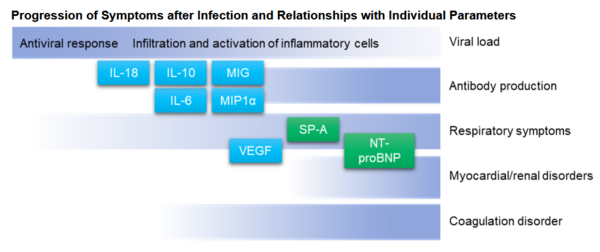
The majority of patients suffer only light symptoms from COVID-19, the infection that occurs when SARS-CoV-2 enters the body. However, many asymptomatic carriers exist, and among otherpatientsthe disease can rapidly grow more severe. In preparation for a predicted resurgence of COVID-19, an urgent need exists for efforts to establish diagnostic tests that cover the time period spanningfromimmediately after infection through to treatment and recovery. This calls for a testing method that accommodates clinical applications, such as the confirmation of initial diagnosis, confirmed diagnosis, risk of increasing severity, and confirmation of treatment effect, and that satisfiesmeasurement parameters and measurement times (number of samples processed).
When a virus enters the body and attacks cells, those cells secrete immunoregulatory proteins called cytokines. By communicating the existence of the virus to immune cells, cytokines help immune cells to destroy cells infected by the virus. In some individuals, excessively active immune cells can begin attacking normal cells and tissues, which is known to injure the lungs and various other organs. In this process, large numbers of cytokines are released into the bloodstream in a phenomenon known as a cytokine storm. Cytokine storms are thought to be related to the increasing severity of COVID-19, and expectations are growing for the elucidation of this mechanism and the practical realization of clinical tests.
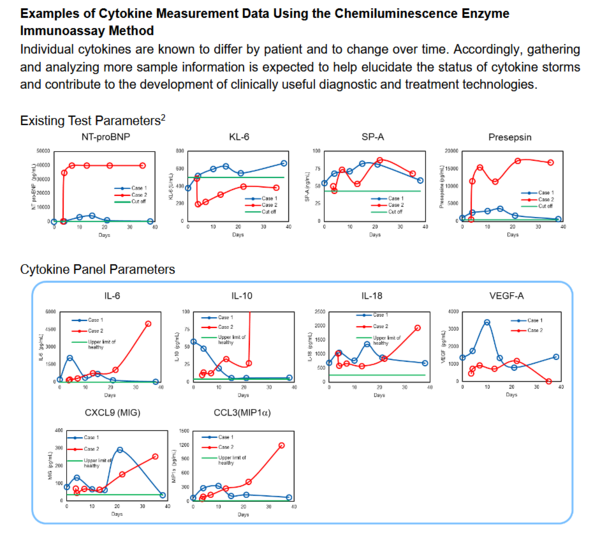
Against this backdrop, on July 1, 2020 Sysmex began providing “cytokine measurement for research,” a cytokine lab assay service for research using the chemiluminescence enzyme immunoassay method. Targeting research institutions, universities, medical institutions and pharmaceutical companies, we will provide data that can be used toconfirm testing methods suitable to such clinical applications as determiningthe risk of increasing severity and treatment effect, as well as data that can be usedfor vaccines, antiviral drugs and other drug discovery research. We will start by providing a lab assay service for research onthe six cytokines most relevant to COVID-19. We will gradually expand the parameters and continue development with a view to providing reagents for research.
Through the provision of a lab assay service for research that leverages its proprietary measurement platforms and the technologies it possesses, Sysmex will continue promoting R&D toward the practical realization of biomarkers with high clinical value, working with customers to help achieve advances in healthcare.
Service Overview
| Name: | Cytokine measurement for research |
| Parameters measured | IL-6: In addition to infection and other acute inflammation, IL-6 is a representative pro-inflammatory cytokine that is deeply connected with the chronic immune disease mechanism. IL-10: This inflammation suppression cytokine is known to play a role in suppressing the Type 1 immune response3to infections. VEGF-A: An important angiogenic factor, VEGF-A is produced during hypoxia. This cytokine acts on endothelial cells and promotes proliferation, the production of intracellular matrices and the acceleration of vascular permeability. IL-18: This pro-inflammatory cytokine is released when cells encounter high levels of stress due to virus infection, cancers, active oxygen or other factors. CXCL9 (MIG): This cytokine governs the Type 1 immune response. Producedby macrophages, epithelial cells and endothelial cells, it migrates to inflammation sites, such as Th1 cells, CD8T cells and certain macrophages. CCL3 (MIP1α): These cytokines are produced in response to inflammation from macrophages, fibroblasts, epithelial cells and vascular smooth muscle cells. |
| Target region: | Japan |
Terminology
1 Huang KJ, Su IJ, Theron M, et al. “An interferon-gamma-related cytokines storm in SARS patients.”J Med Virol. 2005;75(2):185-194. doi:10.1002/jmv.20255Woo PC, Tung ET, Chan KH, Lau CC, Lau SK, Yuen KY. “Cytokine profiles induced by the novel swine-origin influenza A/H1N1 virus: implications for treatment strategies.”J Infect Dis. 2010;201(3):346‐353. doi:10.1086/649785
2 Existing test parameters:These refer to NT-proBNP, KL-6, SP-A and Presepsin.
3 Type 1 immune response (Type 1 immunity):An immune response activated due to macrophages, etc. This response plays a role in defense against viral infection.