Scientific Calendar May 2019
Why is normalisation of LA screening and confirmation results against the local reference interval recommended?
Because it increases the sensitivity of the assay.
Because it increases the specificity of the assay.
Because it increases the accuracy of the patient results.
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific Background
Antiphospholipid syndrome (APS) is a frequent autoimmune disease. It can be ‘primary’, if no other disease is present, or ‘secondary’, when it is associated with an existing disorder. APS is clinically associated with venous and/or arterial thrombotic events and pregnancy complication leading to recurrent foetal loss, growth retardation and premature birth. In rare cases, patients may develop the catastrophic antiphospholipid syndrome (CAPS) characterised by multi-organ thromboses and a high death rate [1].
The clinical spectrum of APS is wide, and it is essential to provide the most appropriate therapeutic management. The diagnosis of APS is confirmed if at least one clinical and one of the laboratory criteria is present. Positive lab test findings must be repeated and confirmed at least 12 weeks after the first finding to prevent false positive reporting [2].
Clinical criteria for APS are summarised in the table below [1].
| Vascular thrombosis | One or more clinical episodes of objectively verified vascular thrombosis |
| Pregnancy morbidity |
|
Laboratory diagnostics involves two approaches mainly to determine the anti-phospholipid antibodies [3, 7, 8]:
- the determination of lupus anticoagulant (LA) by clotting assays.
- the determination of antibodies anti-cardiolipin (aCL) and/or anti-β-2-glycoprotein 1 (aβ2GP1) with specific solid-phase immunoassays.
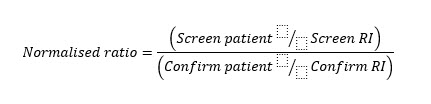
The first line of assays for the laboratory diagnosis of APS is proposed to measure the anticoagulant activity of the antiphospholipid antibodies (aPL). Recent guidelines recommend using two complementary tests: dRVVT and APTT for the diagnosis of LA. Both use low concentrations of phospholipids for screening (e.g. HEMOCLOT™ LA-S or CEPHEN™) and high concentrations of phospholipids for confirmation testing (e.g. HEMOCLOT™ LA-C or CEPHEN™ LS). This test panel can be completed by performing a mixing test with a 1:1 proportion of patient plasma (PP)/normal pooled plasma (NPP) [3, 4, 9, 10]. Ongoing therapy with anticoagulants may interfere with LA testing because the assay principle is based on the competition of the antibodies with vitamin K-dependent coagulation factors for binding sites on anionic phospholipids. Thus, reagents with high sensitivity to LA but less interference by anticoagulants (anti-VKA or DOACs) should be used [5]. Normalisation of results of screening and confirmation assays against the local reference interval (RI) is recommended by testing at least 40 plasmas from healthy individuals without any medication or disease, as patient results vary from laboratory to laboratory depending on differences in clotting assays and devices [6]. Normalised ratios calculated by dividing the clotting time of the patient by the reference interval to reduce inter- and intra-assay variation must be determined for each batch of samples [5]. Since this number of donors might not be available in every laboratory, the verification of the manufacturer reference interval is a reasonable alternative [6]. Generally, positive LA plasmas have a normalised screen/confirm ratio of >1.20, but cut-off values must be determined by each lab (see Fig. 1).
Second line of assays are immunoassays to determine the antibodies (IgM and IgG) aCL and aβ2GP1. Two types of aCL antibodies are relevant: those binding to cardiolipin directly and those binding to cardiolipin in the presence of the co-protein β2GP1. However, only the last one is specific for APS; the first one is found in patients with infectious diseases such as malaria, hepatitis or syphilis. Former anti-cardiolipin/anti-phospholipid antibodies (ACA/APA) assays determined the antibodies against cardiolipin in the presence of bovine β2GP1, which may lead to incorrect results. Nowadays, these types of assays are often replaced by assays with non-oxidised anionic phospholipids and non-denatured human β2GP1 (highly purified), leading to more specific and standardised determination. Specific anti-β2GP1 assays detecting autoantibodies targeting the domain 1 of the specific β2GP1 could offer better specificity at a controlled concentration [5].
Other tests associated with APS are assays to detect antibodies against prothrombin, protein S, protein C, protein Z, annexin V and factor XIII. However, due to a lack of standardisation and evidence about their clinical utility in APS patients, it is not recommended to include these assays in standard test panels.
Basically, only moderate or high concentrations of antibodies are significant for the diagnosis of APS, whilst borderline results require a follow-up to verify the results. Patients with two or more positive results in LA, ACA/APA or β2GP1 antibodies were more strongly associated with thrombotic events or miscarriage than patients who were positive for only one test. Subsequently, it is strongly recommended to perform all three assays at once on the same sample and to repeat testing at least 12 weeks after the initial test.
APL are very heterogeneous, with overlapping reactivities in some patients and not in others. Thus, accurate assays with well-documented performance characteristics are needed for the classification of the antibody in the patient in question. Even though assays are better standardised and more specific and precise, further improvements are expected in the upcoming years [5].
References
[1] Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006 Feb; 4(2):295–306.
[2] Dlott JS. Diagnosing antiphospholipid antibody syndrome: a review of the criterion for definite APS. Urol Nephrol J 2015; 8(Suppl. 1):18–21.
[3] Devreese KMJ. Antiphospholipid antibody testing and standardization. Int Jnl Lab Hem. 2014; 36:352–363.
[4] Ledford-Kraemer MR, Moore GW, Bottenus R, Daniele C, de Groot PG, Exner T, et al. Laboratory testing for the lupus anticoagulant. 1st ed. Approved guideline. CLSI document H60. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2014.
[5] Amiral, Peyrafitte, Dunois, Vissace, Seghatchian, et al. Anti-phospholipid syndrome: Current opinion on mechanisms involved, laboratory characterization and diagnostic aspects. Transfusion and Apheresis Science 56 (2017) 612–625.
[6] Clinical and Laboratory Standards Institute. Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory. 3rd ed. Approved guideline. C28-A3. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2008.
[7] Sangle NA, Smock KJ. Antiphospholipid antibody syndrome. Arch Pathol LabMed 2011; 135:1092–6.
[8] Pengo V, Banzato A, Bison E, Denas G, Zoppellaro G, Bracco A, et al. Laboratory testing for antiphospholipid syndrome. Int J Lab Hematol 2016; 38(May (Suppl. 1)):27–31.
[9] Moore GW, Culhane AP, Daw CR, Noronha CP, Kumano O. Mixing test specific cut-off is more sensitive at detecting lupus anticoagulants than index of circulating anticoagulant. Thromb Res 2016; 139(March):98–101.
[10] Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, de Groot PG. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 2009; 7:1737–40.