The National Cancer Center (Location: Tokyo, Japan; President: Hitoshi Nakagama; “NCC”), the National Center for Global Health and Medicine (Location: Tokyo, Japan; President: Norihiro Kokudo; “NCGM”), and Sysmex Corporation (HQ: Kobe, Japan; Chairman and CEO: Hisashi Ietsugu) are conducting joint research related to an antigen/antibody test for SARS-CoV-2, the virus that causes novel coronavirus (COVID-19). The entities hereby provide an overview of these activities and results of clinical evaluationsof antibody tests. The Japan Health Research Promotion Bureau (JH) supportsthisresearch project throughthe NCC and NCGM.
COVID-19, an infectious disease caused when SARS-CoV-2 sneak intothe body, is highly contagious,symptoms can worsen rapidly, and itis believed that many carriers are asymptomatic. 6.9 million people all over the world have been infected with the disease, 400,000 died,Japan has confirmed 17,000 infections and 900 deaths as of June 8, 2020.1 Currently, the first infectious wave of COVID-19 is converging in Japan, but the infection spread of the successively second wave, the third wave is expected. In preparation for the expected resurgence of COVID-19, we recognize the urgent need to establish a new testing methodology effective throughout from immediately after infection to the treatment and recovery period.
In May 2020, NCC, NCGM, and Sysmex began joint research,clinical evaluations related to an antigen/antibody testing method for SARS-CoV-2. NCC and NCGM providethe samples, and advice on test method development with clinical expertise.Meanwhile, Sysmex is using chemiluminescence enzyme immunoassay to develop diagnostic reagents to detect antigens present in nasopharyngeal mucus and antibodies in the blood (IgM, IgG). Sysmex also measures and analyzes clinical performance, using the samples provided by the two centers. The newly established Japan Health Research Promotion Bureau (JH) is assisting this joint research by offering diagnostic support and assistance towardsmedical implementation.
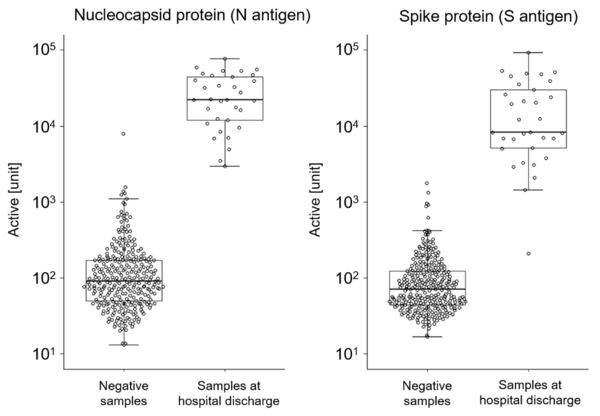
The antibody detection reagents Sysmex has developed are used in conjunction with the in-housefully automated immunoassay system. The reagents detect individual antibodies (IgG, IgM) that react specifically with nucleocapsid protein2 (N antigen) and spike protein3 (S antigen).
Using these reagents,concentrations of IgG antibodies against circulating N antigens and S antigens ina SARS-CoV-2 negative group and a group of SARS-CoV-2 patients upon hospital discharge were compared. Figure 1 shows a clear rise in antibody titersin the patient group, compared with the negative group. Sysmex believes these detection reagents can be used in a broadrange ofepidemiological research, vaccine effect monitoring, and other clinical applications, providing a foundation for futuredevelopment of treatments.
In preparation for an expected resurgence of COVID-19, NCC, NCGM and Sysmex work together expediently establishing a testing method for clinical application, and contributing to diagnosis and treatment of COVID-19.
Sysmex’s Efforts Regarding COVID-19
As a manufacturer of clinical testing instruments and reagents, Sysmex supports ongoing clinical testing conducted in healthcare settings around the world. We believe it is our mission to work alongside healthcare professionals to do everything in our power to prevent the spread of COVID-19 and overcome this crisis. To this end, we are doing our utmost to ensure a stable supply of products and services. Furthermore, we are engaged in initiatives to help prevent COVID-19 from spreading and to allow conditions to return to normal as quickly as possible. On this front, we have obtained the first regulatory approval in Japan for a novel coronavirus testing kit (RT-PCR method). We are leading Japan in collaboration between industry and the public sector (the city of Kobe) to enhance the PCR testing system. In addition, we are working proactively to develop new diagnostic technologies.
https://www.sysmex.co.jp/en/COVID19.html
About the Japan Health Research Promotion Bureau (JH)
Six national R&D centers (NCC, NCGM, the National Cerebral and Cardiovascular Center, the National Center of Neurology and Psychiatry, the National Center for Child Health and Development, and the National Center for Geriatrics and Gerontology) conduct studies, research, and technical development related to advanced medicine. In April 2020, the Ministry of Health, Labour and Welfare and the six centers launched the Medical Research Promotion Division, National Research Center Specializing in Advanced Medicine (JH) to accelerate groundbreaking R&D related to clinical practice. JH decided to support R&D addressing SARS-CoV-2 swiftly and effectively,providing research and clinical testing support to companies participating in this project
Terminology
1 From the “WKC’s new webpage with WHO official information on COVID-19 in Japanese” (As of June 8, 2020) https://extranet.who.int/kobe_centre/en/news/COVID19_specialpage
2 Nucleocapsid protein (N antigen):A protein that constitutes the core structure of a virus, significantly affecting virus characteristics.
3 Spike protein (S antigen):A protein that forms countless protrusions around the virus to bind with cell receptors causinginfection.