CytoCell® IVDR FISH probes
IVDR-certified FISH probes
- Available now! The first IVDR-certified probes on the market
- Safe, reliable and effective products for you and your patients
- Haematological cancers and prenatal conditions
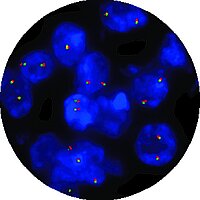
In 1991, CytoCell® became the first provider of FISH probes in the world. Over 30 years later, they are still pioneering the FISH frontiers and were the first to market with In Vitro Diagnostic Regulation (IVDR) certified FISH probes!
Switching to IVDR-certified FISH probes means you can be confident that your laboratory is using safe, reliable and effective tools for testing patients.
CytoCell® IVDR FISH probes remain the same trusted products with the extra IVDR seal — the unquestionable testament to our commitment to provide innovative, class-leading products under this substantially more stringent regulation.
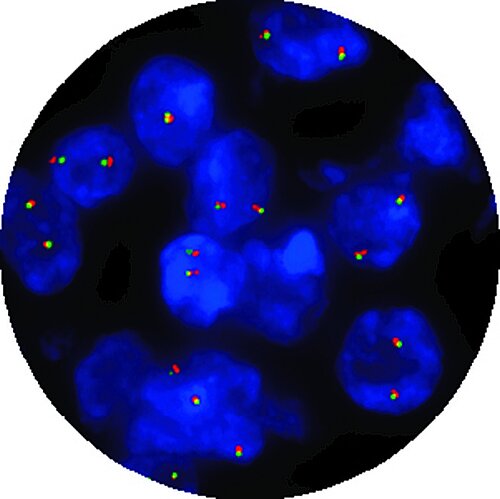
Sixteen CytoCell® FISH probes that are important for the patient management of haematological cancers and prenatal conditions have been IVDR-certified and are available to order. OGT will continue to pursue additional certifications for our CytoCell® FISH probe portfolio.
Switch to a CytoCell® IVDR-certified FISH probe now and be assured that you won’t need to consider it again. Should you need any support, our expert team is always on hand to guide you through the transition.
Start the process early – contact your local Sysmex representative and request an evaluation kit today!
| Haematology probes | Product code |
| AML1 (RUNX1) Breakapart Probe | CE-LPH027 |
| AML1/ETO (RUNX1/RUNX1T1) Translocation, Dual Fusion Probe | CE-LPH026 |
| BCR/ABL (ABL1) Translocation, Dual Fusion Probe | CE-LPH007 |
| BCR/ABL (ABL1) Plus Translocation, Dual Fusion Probe | CE-LPH038 |
| CBFB Breakapart Probe | CE-LPH 089 |
| CBFβ (CBFB)/MYH11 Translocation, Dual Fusion Probe | CE-LPH022 |
| CKS1B/CDKN2C (P18) Amplification/Deletion Probe | CE-LPH039 |
| Del(5q) Deletion Probe | CE-LPH024 |
| Del(7q) Deletion Probe | CE-LPH025 |
| Del(20q) Deletion Probe | CE-LPH020 |
| EVI1 (MECOM) Breakapart Probe | CE-LPH036 |
| FAST PML/RARα (RARA) Translocation, Dual Fusion Probe | CE-LPH064 |
| IGH/MAF Plus v2 Translocation, Dual Fusion Probe | CE-LPH108 |
| MLL (KMT2A) Breakapart Probe | CE-LPH013 |
| P53 (TP53)/ATM Combination Deletion Probe | CE-LPH052 |
| Prenatal probes | |
| Prenatal 13 and 21 Enumeration Probe Kit | CE-LPA003 |
CytoCell® IVDR-certified range of fluorescence in situ hybridisation (FISH) probe kits are in vitro diagnostic (IVD) medical devices for the detection of prenatal trisomy 13 & 21 and acquired cancer-related chromosome alterations.
They have been CE-marked under Regulation (EU) 2017/746 (IVDR) as Class C IVD medical devices for laboratory professional use only and are not intended for use as a standalone diagnostic or companion diagnostic.
Refer to each individual FISH probe kit’s Instructions for use for their specific Intended purpose, indications, and limitations.
| Probe | 50µl per vial (5 tests) or 100µl per vial (10 tests), provided in hybridisation solution and are ready to use. |
| Counterstain | 150µl per vial (15 tests), DAPI antifade |
| Storage temperature | -25°C to -15°C, in the dark |
Sysmex Europe SE
Deelböge 19 D
22297 Hamburg
Germany
+49 (40) 527 26 0
+49 (40) 527 26 100
Product documents
Regulatory documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex