Watmind SARS-CoV-2 antigen immunofluorescence system
Rapid and reliable antigen immunofluorescence system
- Higher analytical performance compared to conventional antigen rapid diagnostic tests
- Portable instrument with built-in printer
- User-friendly and fast operation antigen rapid diagnostic tests
- Standardised and traceable results in 10 minutes
- Prequalified calibration
- QC material available
Perform the rapid test assay with an instrument and get objective, qualitative results within minutes.
Patient information and results can be stored, reviewed, printed, sent or transferred to LIS.
Fast and reliable point-of-care solution
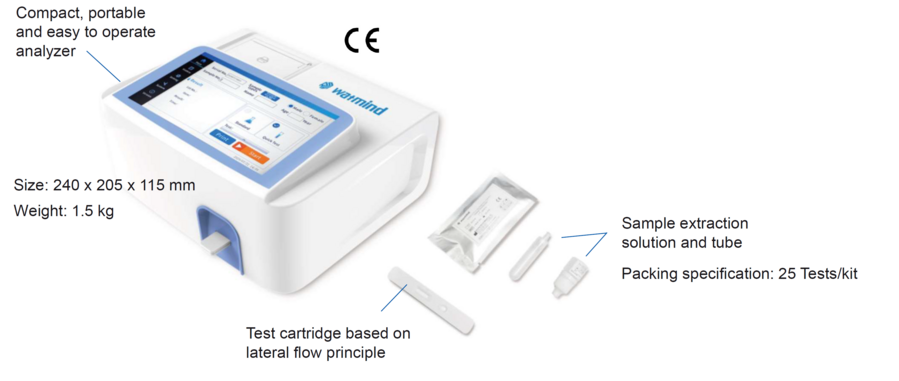
The system consists of a test cartridge, instrument and accessories such as sample collection and QC material.
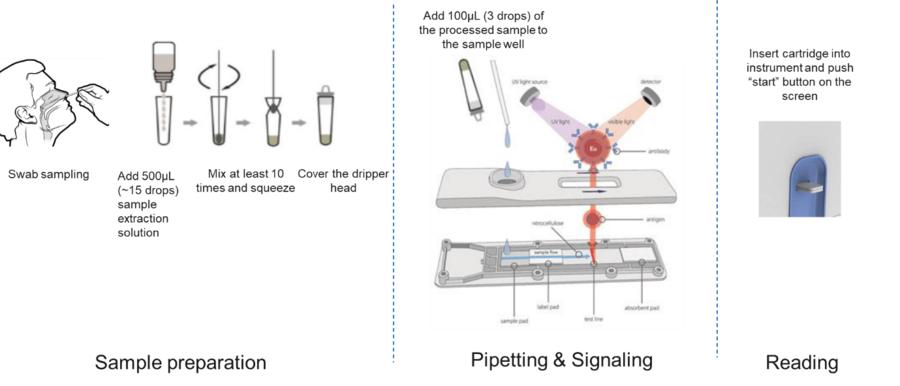
Following the collection of the respiratory specimen, it is applied to the test strip and after the incubation time, results are read via an immunofluorescence instrument.
The use of such a reader standardises and simplifies interpretation of test results between different operators and provides traceable results for documentation.
The easy-to-use and portable point-of-care instrument is based on Europium Fluorescence Technology, providing higher sensitivity and delivering printable results in 10 minutes. The reader can be also connected to an external IT device.
Instrument
| Technology | Immunofluorescence analyser |
| Instrument size | 240*205*115 mm |
| Instrument weight | 1.5 kg |
| Instrument features | 18 cm touch screen, printer, lot ID chip |
| Time to result | 10 minutes |
| Interfaces | USB, COM, Internet (LIS), Wi-Fi |
| Power supply | AC100-240V, 50/60Hz |
Test cassette
| Technology | Lateral flow sandwich immunoassay |
| Sample type | Nasopharyngeal and oropharyngeal swab |
| Buffer volume | 500 µl (15 drops)/ test |
| Sample volume | 100 µl (3 drops)/ test |
| Sensitivity | 90.8% (RT-PCR CT value ≤ 36) |
| Specificity | 99.1% |
| Packaging specification | 25 tests/ kit |
for more details please refer to the IFU
Sysmex Europe SE
Bornbarch 1
22848 Norderstedt
Germany
+49 (40) 527 26 0
+49 (40) 527 26 100
Product documents
Regulatory documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My SysmexExplore more
Thank you very much for your interest in our products. Due to legal restrictions, advertising for medical products that detect COVID-19 infections is not permitted outside of specialist fields. Therefore we ask you to confirm below that you belong to one of the following groups:
- health care professional
- a facility that serves human or animal health
- a person who lawfully works with medicine or medical devices, processes or treatments
This site is for 'Professionals' only. You are not authorised to view this page.