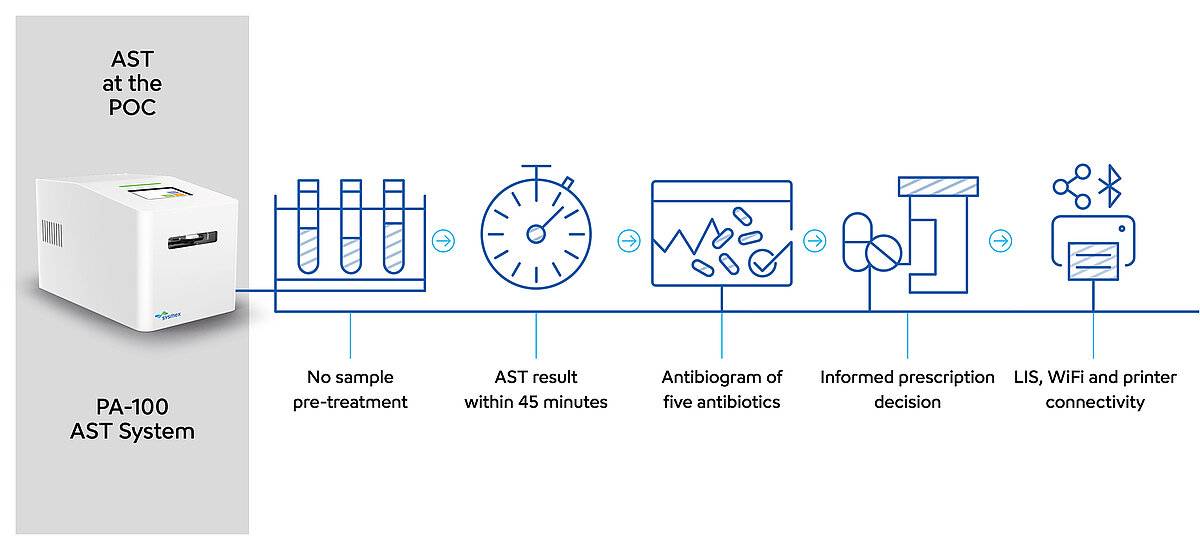
PA-100 AST System
Guiding the future of antibiotic treatment
- Winner of the prestigious £8 million Longitude Prize for AMR research
- Automated and rapid analysis of urine samples
- Phenotypic AST, based on EUCAST standards
- Straightforward: no sample pre-treatment and results during a patient’s first visit
- Targeted antibiotic prescription in less than one hour – reduces risk of AMR
Antibiotic susceptibility testing for near-patient settings
Urinary tract infections (UTI) are one of the most common bacterial infections and also one of the driving causes of antibiotic prescription worldwide. Proper antibiotic prescription is generally achieved by performing urine cultures and antimicrobial susceptibility testing (AST) at the microbiology laboratory.
UTI patients are generally treated by their GP. Due to the limited availability of point-of-care diagnostics, empiric treatment is standard in many settings. Due to the high incidence of the disease, empiric UTI treatment fuels antimicrobial resistance (AMR). [1]
The PA-100 AST System is an automated analyser that combines phase contrast microscopy and nanofluidics to make available antibiograms, for the first time, at the point of care (POC). By using ground-breaking technology, we can achieve the most advanced phenotypic diagnostic performance possible.
Longitude Prize Winner for AMR
In response to the growing public health threat of Antimicrobial Resistance (AMR), often referred to as antibiotic resistance or superbugs, Sysmex’s PA-100 AST System was awarded the prestigious £8 million Longitude Prize on AMR. Established in the 18th century by the British Parliament, the Longitude Prize has a rich history of incentivising scientific breakthroughs to address critical challenges.
Today, the Longitude Prize continues this legacy by focusing on pressing global issues, with the AMR iteration aiming to stimulate the creation of rapid and accurate point-of-care diagnostic tests. The PA-100 AST System aligns perfectly with this goal, functioning as a rapid point-of-care diagnostic test that allows for swift identification of bacterial pathogens and targeted antibiotic selection. This innovation has the potential to improve antibiotic stewardship and mitigate the emergence of AMR.
Rethinking routine antibiotic susceptibility testing
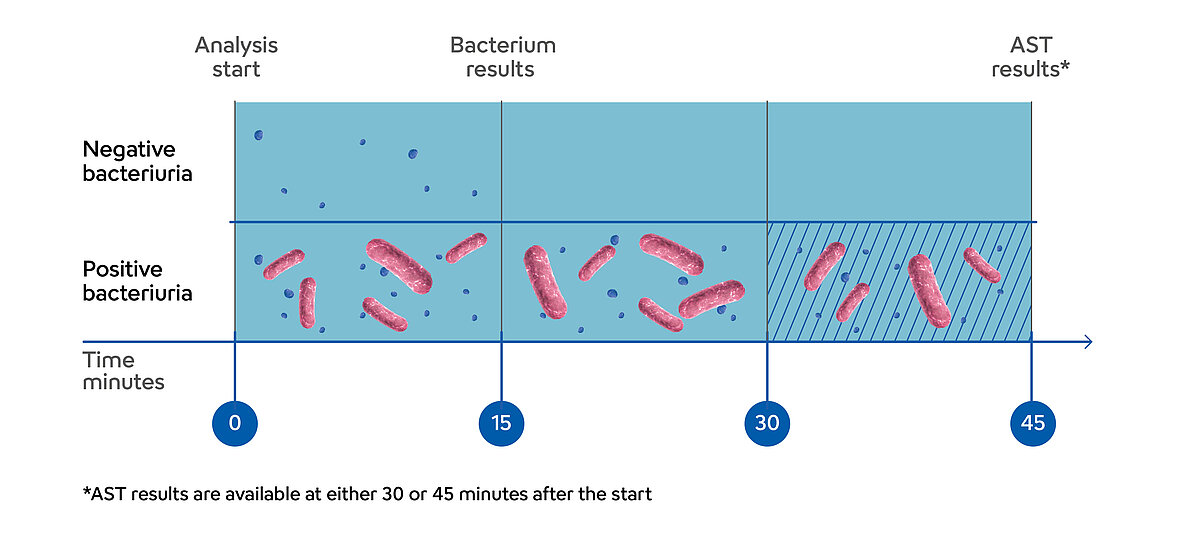
The PA-100 AST System relies on nanofluidics to perform rapid AST. The PA-AST Panel is equipped with a nanofluidic chip, which contains 11.000 nanochannels. The sample is flushed through the chip and single bacterial cells are trapped in individual channels. Larger cellular components are filtered to keep them out of the nanofluidic chip.
Bacteria are incubated in Mueller Hinton Broth II and exposed to various conditions: five different antibiotics, including several concentrations, are tested simultaneously. Cell growth is monitored in real time using contrast phase microscopy. Resistant bacteria keep a higher growth rate during incubation, while susceptible ones grow slowly or lyse. An expert software performs an automatic presumptive species identification of the bacterial strain and provides an easy to interpret antibiogram after assay completion.
This way, the PA-100 AST System provides an easy to use solution. We believe this analyser is suitable for GP practices, emergency departments (ED) and other near-patient settings.
Targeted antibiotic treatment in 45 minutes
Due to the uniqueness of the single cell analysis set up, the PA-100 AST System measures the actual (expressed) resistance profile. This data is analysed to provide an antibiogram based on the phenotypic AST profile of the bacterial strain tested. The results are generated as fast as the biological response of the bacterial strain to the antibiotic.
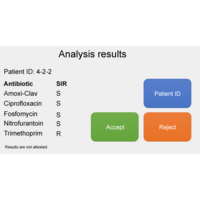
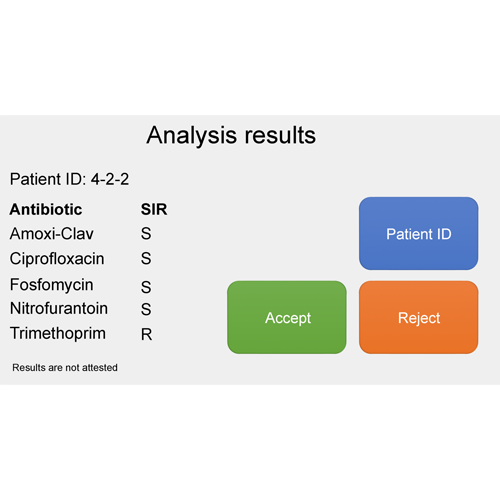
Five commonly prescribed antibiotics for the treatment of uncomplicated UTI are tested in the PA-AST Panel U-0501: amoxicillin/clavulanic acid, ciprofloxacin, fosfomycin, nitrofurantoin and trimethoprim.
The system has been calibrated to evaluate the resistance profile of bacteria based on the EUCAST standards (clinical breakpoint tables). The results are displayed automatically upon assay completion as a regular antibiogram, indicating resistance based on the S/I/R system (Susceptible/Susceptible – Increased exposure/Resistant).
The PA-100 AST System has been evaluated with the most common uropathogenic bacterial species in uncomplicated UTI:
- Gram negative: Escherichia coli, Klebsiella pneumonia, Proteus mirabilis
- Gram positive: Staphylococcus saprophyticus, Enterococcus faecalis
AST at the POC
The PA-100 AST System has been designed to provide a user-friendly solution for the POC.
Unlike the conventional AST workflow in urinalysis, this system does not require a urine culture previous to the AST. Native, fresh urine is directly pipetted into the PA-AST Panel U-0501. No sample pre-treatment is required, making the PA-100 AST System suitable for POC settings.
Support your UTI diagnostic decisions and antibiotic treatment selection, with minimum hands-on time and no microbiology training. All this right at the point of need, outside of the microbiology laboratory environment.
References
[1] Friedman ND, Temkin E, Carmeli Y. (2016): The negative impact of antibiotic resistance. Clinical Microbiology and Infection, 22(5), 416-422.
[2] World Health Organization. (2016): Diagnostic stewardship: a guide to implementation in antimicrobial resistance surveillance sites (No. WHO/DGO/AMR/2016.3).
The PA-100 System comprises two main elements
| Main analyser | PA-100 |
| Measurement principle | Phase contrast microscopy |
| Sample volume | 400 µL |
| Time to result |
|
| Display | 4,3” Resistive Touch Screen LCD |
| Maintenance | Maintenance-free |
| Memory | 500 results (including AST) |
| Quality control | System performance: PA Control Beads |
| Connectivity | USB, External printer (optional) LIS (POCT1-A) |
| Accessories | Barcode reader (included) WiFi dongle (optional) |
| Dimensions | 200 x 220 x 360 mm (W x H x D) |
| Weight | 13 kg |
Single use cartridge
| Single use sample cartridge | PA-AST Panel U-0501 |
| Sample type | Fresh native urine (not older than 30 minutes) |
| Sample volume | 400 µL |
| Bacterial growth medium | Mueller Hinton Broth II |
| Tested on five bacterial species | E. coli, P. mirabilis, K. pneumoniae, S. saprophyticus, E. faecalis |
| Antibiogram for five antibiotics | Amoxicillin/clavulanic acid, ciprofloxacin, fosfomycin, nitrofurantoin, trimethoprim |
Sysmex Europe SE
Deelböge 19 D
22297 Hamburg
Germany
+49 (40) 527 26 0
+49 (40) 527 26 100
Growing your knowledge
We offer all our scientific and operational expertise with Caresphere Academy, a unique portal for all your training objectives. Just sign up, browse through our catalogue and find the courses you need in the blink of an eye.
Product documents
Regulatory documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex