CALiaGold
Quantitative calprotectin measurement on your dedicated stand-alone faecal analyser
- Home sampling with improved ease of use
- Simplified laboratory workflow
- Just one step from sample collection to analysis
- No contamination risk in the laboratory
- Fast and fully automated quantitative immunoassay
Calprotectin is a protein mainly found in the cytoplasm of neutrophilic granulocytes. In cases of gastrointestinal (GI) inflammation, neutrophils migrate through the intestinal wall and calprotectin is released due to degranulation. It accumulates in the faeces and is excreted from the body. The concentration of calprotectin directly correlates with the number of neutrophils in the intestinal lumen, making it an ideal biomarker for GI inflammation.
The determination of faecal calprotectin (fCal) is a non-invasive option to assess localised inflammation. Being highly sensitive for the detection of GI inflammation, it helps in the differential diagnosis of inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS). The biomarker fCAL outperforms general inflammation markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) since it is more sensitive and more specific.
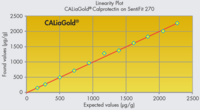
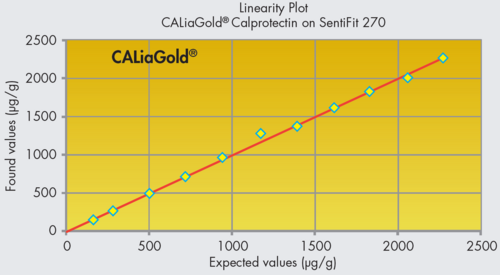
CALiaGold is the complete solution for the automated quantitative determination of calprotectin in human faeces. The particle-enhanced turbidimetric immunoassay (PETIA) is based on the agglutination reaction between latex particles coated with antibody and the antigen in solution. It can be run, along with the FOB Gold test, on your dedicated faecal analyser (SENTiFIT 270 Analyser or SENTiFOB Analyser).
The CALiaGold system comprises a tube for sample collection and a next-generation immunological latex reagent for the detection of calprotectin. CALiaGold brings true convenience to patients, clinicians, and laboratory professionals.
Easy for the patient, safe and hygienic for the lab – the CALiaGold pierceTube
- Opens only on one side and comes with clear instructions for the patient – for correct sample collection at home
- Standardised sample collection (10 mg faeces)
- The pierceable lid is secured by a tight seal
- High sample stability in the buffer (3 days at up to 37°C, 14 days at 2–8°C)
- No extraction steps or centrifugation
Fast and reliable – the CALiaGold test
- Highly sensitive, quantitative determination of faecal calprotectin
- Particle-enhanced turbidimetric immunoassay (PETIA) of high precision and reproducibility
- Ready-to-use liquid reagents, calibrator, and control
- Immunoparticles coated with avian antibodies against human calprotectin
- Time to first result on the SENTiFIT 270 Analyser: 12 minutes
- Time to first result on the SENTiFOB Analyser: 14 minutes
- Can be performed along with the FOB Gold test on the same faecal analysis system – SENTiFIT 270 Analyser or SENTiFOB Analyser
SENTiFIT®, SENTiFOB and CALiaGold® are trademarks in various jurisdictions, which are exclusively licensed to Sentinel CH. SpA. www.sentineldiagnostics.com
Clinical value
Differential diagnosis of IBD and IBS
- Negative fCAL: low probability of having bowel inflammation (high negative predictive value, NPV), excludes IBD as a diagnosis.
- Elevated fCAL: consistent with the presence of GI inflammation, needs further investigation with endoscopy and histology. No single test is specific enough to diagnose or rule out IBD.
Disease activity
- Normalisation of fCAL levels can be an indicator of effective treatment and achievement of mucosal healing.
Prognostic
- Rising fCAL can predict an imminent clinical relapse of IBD, allowing prompt initiation of treatment.
fCAL
- can be effective for the management of colonoscopy resources,
- demonstrates to be a non-invasive and accurate assay for monitoring disease activity and response to treatment, and
- can be a prognostic tool for clinical relapses.
| Sample stability | 3 days at up to 37°C, 14 days at 2–8°C |
| Test tube | CALiaGold pierceTube |
| Reagents | SENTiFIT 270 Analyser: CALiaGold (R1 and R2); CALiaGold Sample diluent SENTiFOB Analyser: CALiaGold RV (R1 and R2); CALiaGold Sample diluent |
| QC and calibrator | CALiaGold Control Set (2 levels); CALiaGold Calibrator |
| Analysis systems | SENTiFIT 270 Analyser |
Sysmex Europe SE
Deelböge 19 D
22297 Hamburg
Germany
+49 (40) 527 26 0
+49 (40) 527 26 100
Product documents
Regulatory documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex